Concept of serial dilutions (undil, 1:1, 1:2) Last dilution with positive reaction = titer Measures immunologic response, AB concentration Higher titers indicate recent/current infection Used to: Establish baseline titer/immunity Clinical significance of titers (e.g., CF) Monitor treatment success ( 4-fold drop) 2 tube vs 4-fold rise/drop in titer. Serial Vs Parallel Dilution SECOND: Also use a serial dilution when the dilution factor is so large that the amount of stock solution needed to make the dilution in one step (using the formula C 1V 1 = C 2V 2) is too small to measure accurately. Remember that the smallest volume you can measure with the micropipettors is 2 μL.
- Posted in:Admin
- 13/04/18
Contents • • • • Parallel Data [ ] The parallel port on modern computer systems is an example of a parallel communications connection. The parallel port has 8 data wires, and a large series of ground wires and control wires. IDE hard-disk connectors and PCI expansion ports are another good example of parallel connections in a computer system. Serial Data [ ] The serial port on modern computers is a good example of serial communications. Serial ports have either a single data wire, or a single differential pair, and the remainder of the wires are either ground or control signals. USB, FireWire, SATA and PCI Express are good examples of other serial communications standards in modern computers.
Serial Dilution in Microbiology: Calculation. An example serial dilution using the easiest method. In Microbiology: Calculation, Method & Technique. Dilutions: Explanations and Examples of Common Methods. There are many ways of expressing concentrations and dilution. Autocad Lisp Steel Sections Properties there. Serial Dilutions.
Which is Better? [ ] It is a natural question to ask which one of the two transmission methods is better.
Serial And Parallel Dilution
At first glance, it would seem that parallel ports should be able to send data much faster than serial ports. Logitech Mk300 Driver Vista more. Let's say we have a parallel connection with 8 data wires, and a serial connection with a single data wire.
Simple arithmetic seems to show that the parallel system can transmit 8 times as fast as the serial system. However, parallel ports suffer extremely from inter-symbol interference (ISI) and noise, and therefore the data can be corrupted over long distances. Also, because the wires in a parallel system have small amounts of capacitance and mutual inductance, the bandwidth of parallel wires is much lower than the bandwidth of serial wires. We all know by now that an increased bandwidth leads to a better bit rate.
We also know that less noise in the channel means we can successfully transmit data reliably with a higher Signal-to-Noise Ratio, SNR. If, however, we bump up the power in a serial connection by using a differential signal with 2 wires (one with a positive voltage, and one with a negative voltage), we can use the same amount of power, have twice the SNR, and reach an even higher bitrate without suffering the effects of noise. USB cables, for instance, use shielded, differential serial communications, and the USB 2.0 standard is capable of data transmission rates of 480Mbits/sec! In addition, because of the increased potential for noise and interference, parallel wires need to be far shorter than serial wires. Consider the standard parallel port wire to connect the PC to a printer: those wires are between 3 and 4 feet long, and the longest commercially available is typically 25 meter(75 feet). Now consider Ethernet wires (which are serial, and typically unshielded twisted pair): they can be bought in lengths of 100 meters (300 feet), and a 300 meters (900 feet) run is not uncommon!
UART, USART [ ] A Universal Asynchronous Receiver/Transmitter (UART) peripheral is used in embedded systems to convert bytes of data to bit strings which may be transmitted asynchronously using a serial protocol like RS-232. A Universal Synchronous/Asynchronous Receiver/Transmitter (USART) peripheral is just like a UART peripheral, except there is also a provision for synchronous transmission by means of a clock signal which is generated by the transmitter.
• On the use of the serial dilution culture method to enumerate viable phytoplankton in natural communities of plankton subjected to ballast water treatment • There are two situations where serial dilutions should be used rather than parallel dilutions: FIRST: Use a serial dilution when you need several solutions of the same solute and there is a constant dilution factor. Therefore, this series has a constant dilution factor of 10. • THE INDICATOR DILUTION METHOD: ASSUMPTIONS AND APPLICATIONS TO BRAIN UPTAKE Olaf B. Paulson and Marianne M. Hertz State University Hospital, Copenhagen, Denmark • ARQ 197 (Tivantinib) is a novel and selective human c-Met receptor tyrosine kinase inhibitor with a minmal IC50 of 0.1 μM. Find all the information about ARQ 197.
Introduction
A Serial dilution is a series of dilutions, with the dilution factor staying the same for each step. The concentration factor is the initial volume divided by the final solution volume. The dilution factor is the inverse of the concentration factor. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; this has a concentration of 1/10th (0.1) of the original and a dilution factor of 10. These dilutions are often used to determine the approximate concentration of an enzyme (or molecule) to be quantified in an assay. Serial dilutions allow for small aliquots to be diluted instead of wasting large quantities of materials, are cost-effective, and are easy to prepare.
Equation 1.
[concentration factor= frac{volume_{initial}}{volume_{final}}nonumber]
[dilution factor= frac{1}{concentration factor}nonumber]
Key considerations when making solutions:
Serial Dilution Chemistry
- Make sure to always research the precautions to use when working with specific chemicals.
- Be sure you are using the right form of the chemical for the calculations. Some chemicals come as hydrates, meaning that those compounds contain chemically bound water. Others come as “anhydrous” which means that there is no bound water. Be sure to pay attention to which one you are using. For example, anhydrous CaCl2has a MW of 111.0 g, while the dehydrate form, CaCl2 ● 2 H2O has a MW of 147.0 grams (110.0 g + the weight of two waters, 18.0 grams each).
- Always use a graduate cylinder to measure out the amount of water for a solution, use the smallest size of graduated cylinder that will accommodate the entire solution. For example, if you need to make 50 mL of a solution, it is preferable to use a 50 mL graduate cylinder, but a 100 mL cylinder can be used if necessary.
- If using a magnetic stir bar, be sure that it is clean. Do not handle the magnetic stir bar with your bare hands. You may want to wash the stir bar with dishwashing detergent, followed by a complete rinse in deionized water to ensure that the stir bar is clean.
- For a 500 mL solution, start by dissolving the solids in about 400 mL deionized water (usually about 75% of the final volume) in a beaker that has a magnetic stir bar. Then transfer the solution to a 500 mL graduated cylinder and bring the volume to 500 mL
- The term “bring to volume” (btv) or “quantity sufficient” (qs) means adding water to a solution you are preparing until it reaches the desired total volume
- If you need to pH the solution, do so BEFORE you bring up the volume to the final volume. If the pH of the solution is lower than the desired pH, then a strong base (often NaOH) is added to raise the pH. If the pH is above the desired pH, then a strong acid (often HCl) is added to lower the pH. If your pH is very far from the desired pH, use higher molarity acids or base. Conversely, if you are close to the desired pH, use low molarity acids or bases (like 0.5M HCl). A demonstration will be shown in class for how to use and calibrate the pH meter.
- Label the bottle with the solution with the following information:
- Your initials
- The name of the solution (include concentrations)
- The date of preparation
- Storage temperature (if you know)
- Label hazards (if there are any)
Lab Math: Making Percent Solutions
Equation 2.
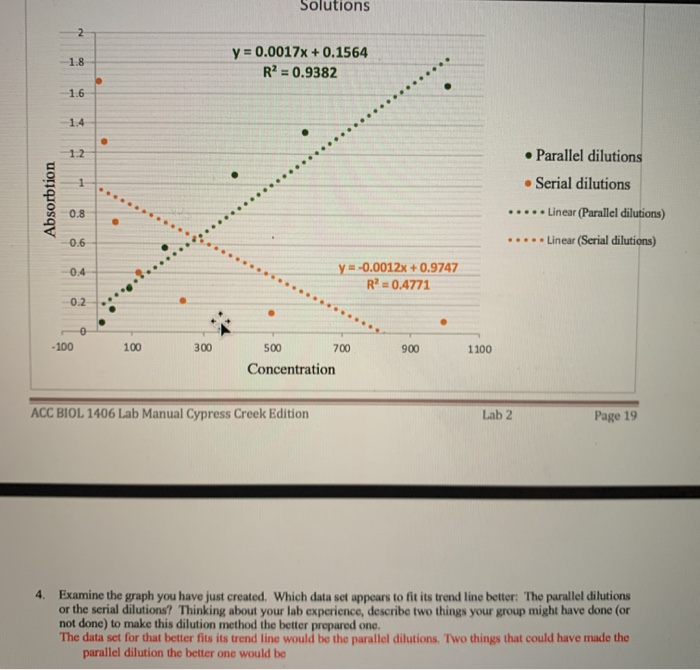
Serial Vs Parallel Dilution
Formula for weight percent (w/v):
[ dfrac{text{Mass of solute (g)}}{text{Volume of solution (mL)}} times 100 nonumber ]

Example
Serial Dilution Problems
Make 500 mL of a 5% (w/v) sucrose solution, given dry sucrose.
Serial Dilution Vs Parallel Dilution
- Write a fraction for the concentration [5:%: ( frac{w}{v} ): =: dfrac{5: g: sucrose}{100: mL: solution} nonumber]
- Set up a proportion [dfrac{5: g: sucrose}{100: mL: solution} :=: dfrac{?: g: sucrose}{500: mL: solution} nonumber]
- Solve for g sucrose [dfrac{5: g: sucrose}{100: mL: solution} : times : 500 : mL : solution : = : 25 : g : sucrose nonumber]
- Add 25-g dry NaCl into a 500 ml graduated cylinder with enough DI water to dissolve the NaCl, then transfer to a graduated cylinder and fill up to 500 mL total solution.